Carbonic acid is a mineral derived from limestone or marl rocks. It is an oxyacid with a formula and uses similar to sodium bicarbonate. In the human body, it serves to increase the acidity of bodily fluids, and in industry, it is used as an ingredient in carbonated beverages.
Contents
Formula of carbonic acid
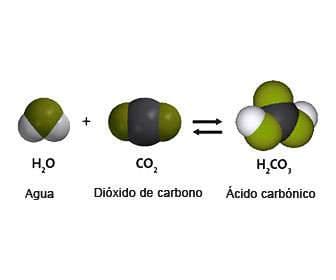
Depending on whether it is diluted or not, it changes due to the loss of 1 or 2 protons. The formula of carbonic acid is:
- H2CO3
When it loses one proton, it turns into bicarbonate, forming hydrogen carbonate ion. Its formula is:
- H2CO3 → HCO3– + H+ (pKd = 6.35)
When it loses its second proton, the result is:
- HCO3– → CO32- + H+ (pKd = 10.33)
Uses and applications
- Carbonated beverages.
- Carbonated soft drinks.
- To produce carbonates with other minerals and supplements.
- To prepare lemonades.
- Effervescent tablets.
- Gardening as a preparation to maintain environmental quality.
- Arc welding.
- As a component of fire extinguishers.
- For refrigeration chambers.
- Used as dry ice.
It is widely used in various industrial and medical sectors, so its applications vary depending on its use. Likewise, the safety data sheet for carbonic acid, depending on whether it is for human consumption or as a fire extinguisher or refrigerant, may slightly vary in its structure.
Carbonic acid in the human body
It is a key element for organic life that develops lungs. Carbonic acid in the human body plays a crucial role in bodily fluids. It is present in extracellular fluids.
Its properties make it ideal as a biological buffer, meaning it helps regulate pH. In the blood, it prevents pH fluctuations through various chemical reactions.
It is a buffering solution for the mixture of acids, maintaining pH stability. This makes it essential for human health.
- When formed from CO2 and water, it works to lower blood pH. This is possible due to its dissolution into H+ and HCO3.
- It is quickly eliminated from the human body due to its volatile properties. CO2 is expelled through respiration.
It is an inert gas, so it is also used in medicine, for example, in laparoscopic surgery. In this procedure, carbonic acid is insufflated into the peritoneal cavity, allowing the abdomen to be examined.
When ingested through a beverage, much of its content is expelled through pulmonary respiration.
Produce Intestinal Gases or Flatulence
It is true that a large amount of it disappears when the bottle containing the beverage is opened. However, the small amount that reaches the intestines can be quite bothersome, and in many cases, it is necessary to resort to home remedies for intestinal gases. Some people may benefit from taking fennel seeds for this purpose.
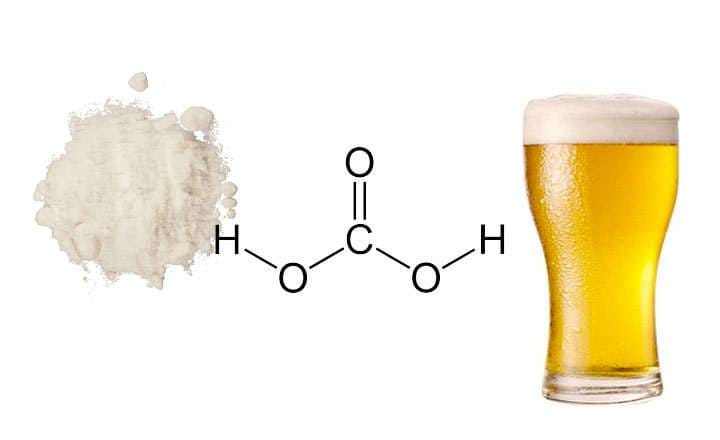
Carbonic Acid and Beer
Uses of carbonic acid in beer cause it to be carbonated. This is visible when the bottle is opened, removing the cap or the cork, and it forms foam.
While the beverage is open, it continues to volatilize as an inert gas and produces bubbles in the liquid.
Baking Soda

Just like carbonic acid, baking soda is effective in regulating the body’s pH, although it is advisable not to overuse it. One tablespoon can be taken daily for several weeks. If used for a longer period, it should be advised by a doctor to avoid alkalizing the body.
Properties and Chemical Formula
- IUPAC Name: Trioxocarbonic Acid (IV)
- Alternative Names: Hydrogen Trioxocarbonate (IV)
- Chemical Formula: H2CO3
- Color: Colorless
- Density: 1000 kg/m3; 1 g/cm3
- Molar Mass: 62.03 g/mol
- Melting Point: K (-273.15 °C)
- Boiling Point: K (-273.15 °C)

i loved it